Kelaniya University clinical trial expertise contributes to the global effort against dengue
Dengue is the most common infectious disease transmitted by a mosquito and is a serious global burden. Between 100–200 million infections occur each year in more than 100 countries, resulting in about 20,000 deaths. It is a major public health problem in Sri Lanka. Explosive epidemics predominantly affecting areas that have high annual rainfall occur once every few years in the background of endemic transmission. During the last decade, severe dengue has become a leading cause of hospitalization and death, especially among children and elderly. In 2017 more than 186,000 people were affected by the disease, eventually killing 440 people. Last year, 31,162 cases were reported from our country.
Increased rates of hospitalization due to severe dengue, during outbreaks, result in massive economic losses and strained health services. Dengue is estimated to cause a global economic burden of USD 8.9 billion per year. In Sri Lanka, direct cost of dengue control and outbreak response activities during the 2017 epidemic alone was estimated to be US $12.7 million, corresponding to a per capita cost of US $0.64.
There is no specific treatment for dengue fever at present. In the absence of specific antiviral therapy, control of transmission of dengue by vector (mosquito) management is the sole method available for decreasing dengue-associated morbidity and mortality. Since vector control strategies alone have not been able to satisfactorily achieve reduction in viral transmission, the implementation of a safe, efficacious, and cost-effective dengue vaccine is a high public health priority.
Development of a dengue vaccine through clinical research
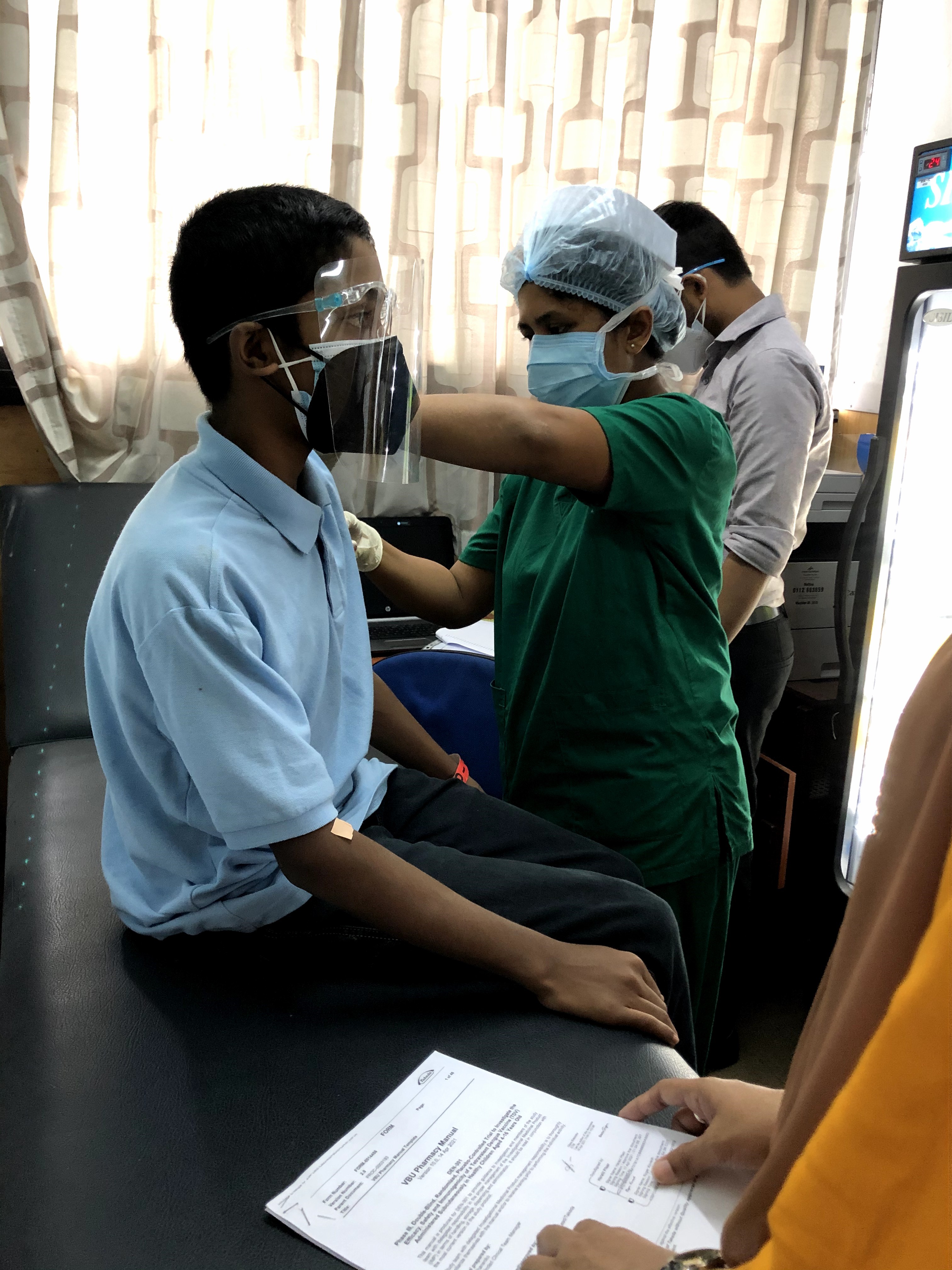
The efficacy, safety, and immunogenicity of a two-dose candidate dengue vaccine TAK-003 manufactured by Takeda Vaccines, Inc. are currently being assessed in a large, multi-centre, phase III, clinical trial (Efficacy, Safety and Immunogenicity of Takeda’s Tetravalent Dengue Vaccine in Healthy Children [TIDES]) involving more than 20,000 children and adolescents 4 to 16 years of age living in Latin America and Asia. TAK-003 is a tetravalent vaccine and is based on a live-attenuated dengue serotype-2 virus (DENV-2) that provides the genetic backbone for protection against all four dengue serotypes.
Sri Lanka is contributing to the global effort to develop a safe and effective vaccine against dengue by participating in this international TIDES clinical trial through the scientific leadership provided by the Clinical Trials Unit (CTU), Faculty of Medicine, University of Kelaniya and its corporate clinical research project management partner, RemediumOne.
The conduct of this pivotal clinical trial in Sri Lanka at three University Paediatric Units and the National Centre for Clinical Management of Dengue under the leadership of consultant paediatricians is being coordinated by the CTU and RemediumOne. The clinical research partnership established between the University of Kelaniya and RemediumOne through a unique public-private partnership provides the required scientific and project management support to conduct the clinical trial at all four centres in Sri Lanka to the highest international standards. 2100 children aged 4-16 years from dengue endemic areas have been enrolled into this clinical trial in Sri Lanka. The study is now in its fifth year of follow up.
Retention of study participants over a period of 5+ years for safety and efficacy follow up is one of the most challenging aspects of the clinical trial. Using several retention strategies including health education programmes, weekly telephonic contact of all 2100 study participants as well as active post-vaccination safety surveillance, research coordinators have successfully retained more than 98% at five years post-vaccination, the highest across all participating centres in the world.
Primary safety and efficacy findings from the first part of this ongoing clinical trial published in the New England Journal of Medicine in November 2019 reported the overall vaccine efficacy against virologically confirmed dengue caused by any serotype was 80.2% and was 95.4% against dengue leading to hospitalization. Vaccine efficacy was highest against DENV-2 (97.7%), the serotype most prevalent in Sri Lanka, while efficacy was modest (62.6 to 73.7%) against the other three serotypes. No serious adverse vaccine-related events were observed.
More recently, results from a 36-month follow-up exploratory analysis of the TIDES trial presented at the 17th Conference of the International Society of Travel Medicine showed the dengue vaccine (TAK-003) demonstrated continued protection against dengue illness and hospitalization, regardless of an individual’s previous dengue exposure, with no important safety risks identified through three years after vaccination in the ongoing pivotal Phase III trial.
These results have clearly shown that vaccine-induced dengue antibody titers remained elevated in all vaccine recipients, regardless of baseline serostatus at 48 months, and suggest TAK-003 vaccine could help with outbreak prevention, reduce rates of hospitalization, protect people from dengue regardless of their previous exposure, and ultimately help address the significant global burden of dengue.
RemediumOne and the CTU have provided scientific leadership to conduct this important clinical trial through training of research teams, project management, provision of periodic quality assessments, supervision of protocol-related activities while adhering to strategic timelines. Global key opinion leaders in vaccine development have applauded Sri Lanka’s involvement as a key stakeholder in this pivotal dengue vaccine project. The efforts of Investigators and RemediumOne to generate high quality data and very high retention rates for long-term follow up to strengthen the integrity of the trial have been highlighted at many international scientific fora.