Contributions for Healthcare services, Research, Development and Training
I. Establishment of an operational model to control dengue in Sri Lanka
Development of modified dengue vector mosquitoes and identification of biological control agents
- Development and semi-field testing of transgenic Aedes aegypti with resistance to dengue viruses (2 & 4).
- Establishment of Sterile Insect Technology (SIT) followed by development of sterile male Aedes mosquitoes and successful field testing.
- Identification of lavivorous fish species and carnivorous copepods species for dengue control.
Personal protective measures using nanotechnology
- A natural oil derived nano particles-based mosquito repellent with activity last for 7 hours developed and ready for commercialization
Repellent
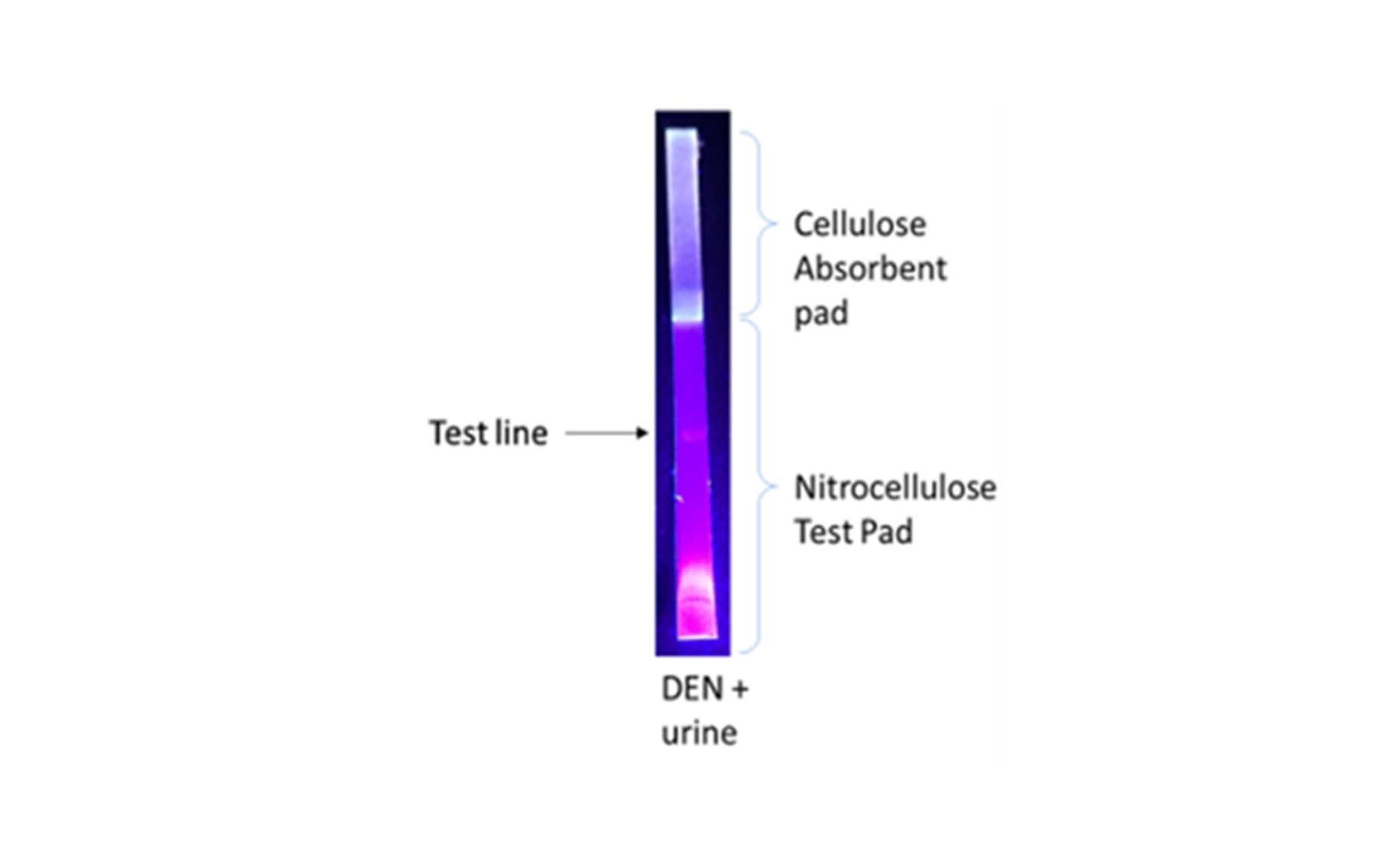
- A Monoclonal antibody (Mab) and a nanoparticle (CdTe Qdots) produced, nanoparticles anchored with the Mab and Lateral Flow Immune Assay (LFIA) developed.
Lateral flow immuno assay - strip
- Risk factors associated with transmission of dengue in high risk areas identified.
- A model for environmental management and community mobilization for the control of dengue in schools developed.
- Knowledge on environmental management and community mobilization for control of dengue among stakeholders and community developed.
- Temporal risk models with prediction power of 3-6 months in advance developed.
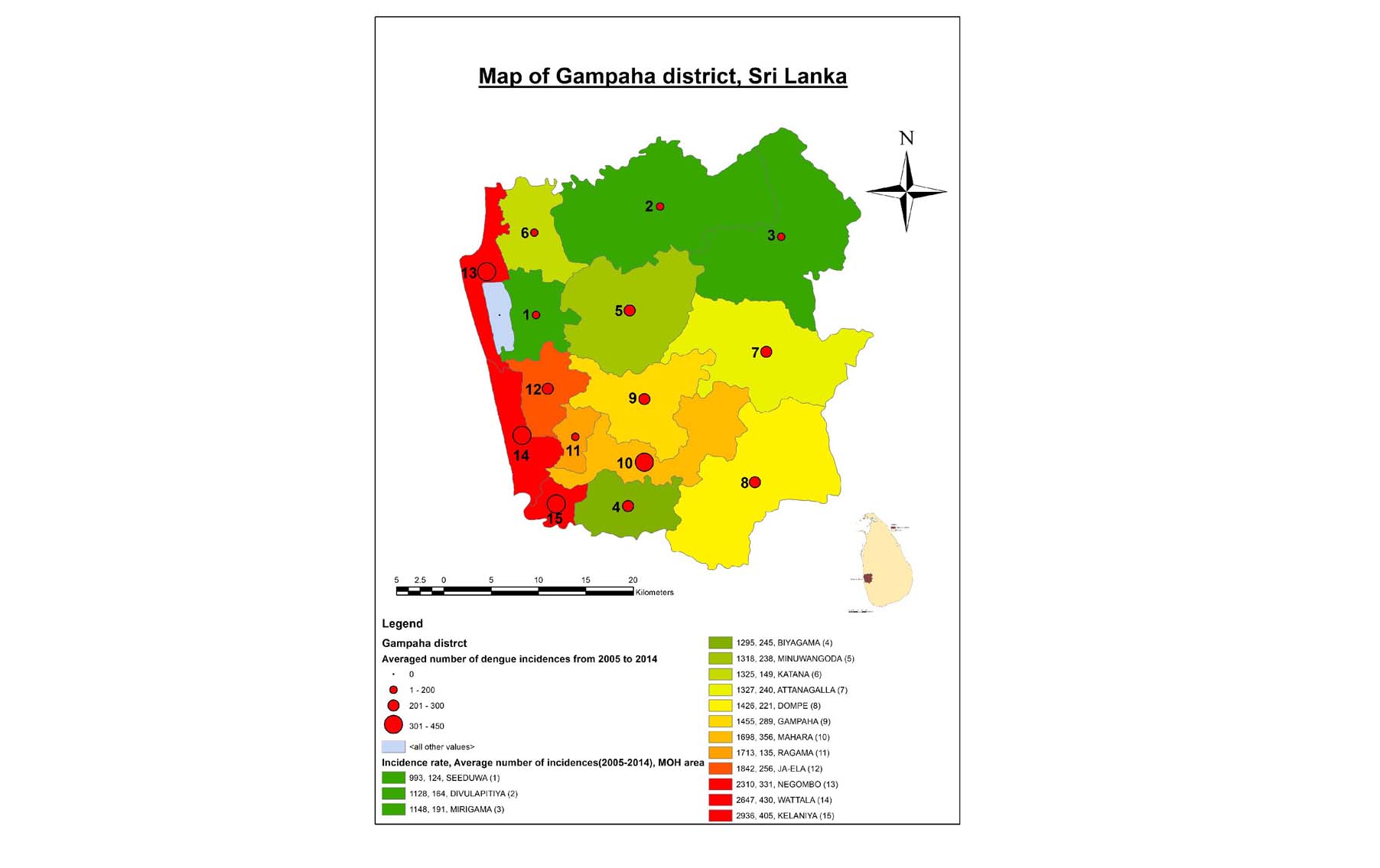
- Spatial risk models with prediction power of 3 months in advance developed.
Risk map for Gampaha District
- All the techniques developed were transferred to the main Ministry of Health.
II. Development of institutional capacity for healthcare services, research and training by upgrading infrastructure
- Insectary for rearing of Aedes mosquitoes
- Arthropod Containment Laboratory (ACL-2)
- Medical entomology laboratories
- Information centre
- Entomology museum
IV. Provision of diagnostic services to the general public
- RT-PCR based testing for qualitative detection of dengue viral RNA
- PCR based testing for qualitative detection of Microbacterium tuberculosis DNA
- PCR based testing for qualitative detection of Hepatitis B virus (HBV) DNA
- Nested-Multiplex-PCR based testing for genotyping of HBV
- Real time PCR based testing for quantification of HBV load
- RT-PCR based testing for qualitative detection of Hepatitis C virus (HCV) RNA
- Nested-multiplex-PCR based testing for genotyping of HCV
- PCR-based testing for qualitative detection Leptospira DNA
- NS1 antigen test for dengue
V. Application of innovative technology-based interventions
We offer the following molecular/other diagnostic testing services at the Molecular Medicine Unit
- RT-PCR based testing for qualitative detection of dengue viral RNA
- PCR based testing for qualitative detection of Microbacterium tuberculosis DNA
- PCR based testing for qualitative detection of Hepatitis B virus (HBV) DNA
- Nested-Multiplex-PCR based testing for genotyping of HBV
- Real time PCR based testing for quantification of HBV load
- RT-PCR based testing for qualitative detection of Hepatitis C virus (HCV) RNA
- Nested-multiplex-PCR based testing for genotyping of HCV
- PCR-based testing for qualitative detection Leptospira DNA
VI. Supporting the national surveillance system for dengue
Five high dengue risk areas in the District of Gampaha were monitored and transferred data to the national surveillance system for 3 years.
VII. Recognition as the national centre representing regional and inter-regional network on development of SIT for integrated control of dengue vectors
VIII. Evaluation of commercial products sent by the Government institutions
Before approval of novel commercial products/agents for mosquito vector control, they need to be evaluated locally. MMU has been identified as a centre for evaluation such products by the Ministries of Health and Environment.
IX. Provision of DNA finger printing services to the judicial services
With the increase in burglary, crime and dispute paternity in the country, the demand for DNA typing services was increasing. Therefore, Molecular Medicine Unit, Faculty of Medicine, University of Kelaniya, Sri Lanka started providing DNA typing services in 2005 as the first government institution providing this service.
- Resolving forensic case work by DNA fingerprinting technology
- Resolving disputed paternity/maternity cases by DNA fingerprinting technology
X. Facilitating post graduate research training opportunities for young scientists
- D- 05 (Completed); 06 (On going)
- Phil- 03
XI. Providing expertise/services consultancy to healthcare and other institutions
- Ministry of Health -02
- Ministry of Environment-02
- Sri Lanka Institute of Biotechnology (SLIBTEC) -01
- Sri Lanka Accreditation Board -01